Why Health Testing Is Important
We often get asked why health testing is so important. If you just want a family companion, does it really matter that much? Our answer is a resounding yes! Whether you’re buying for the show or performance ring or simply want a lifelong friend, health screenings are one of the key factors potential owners should consider before choosing a breeder.
The German Shorthaired Pointer is a fairly healthy breed but, as with any breed, some issues do pop up. Buyers should always inquire about the health clearances of the sire and dam when looking for a puppy. Breeders should have no problem discussing health issues that have occurred in their dogs or lines, or answering questions about the health of the breed as a whole. It is important to understand that there are no perfect dogs, but through health testing and careful pedigree research we can do our very best to avoid genetic health issues in the dogs we produce. It is our obligation as responsible breeders to do everything in our power to ensure we are breeding healthy, long-lived examples of our breed.
Health testing entails much more than simply having our family veterinarian examine our dogs. It includes both genetic screening and examinations by board certified veterinary specialists. These tests look at both “phenotype” — diseases we can test for with X-rays, blood or other physical exam — and “genotype” — those diseases identified by DNA testing. Breeders and puppy buyers should always ask for copies of the test results or look at the OFA website for more detailed information on the results of the tests. You can search the OFA database by going to their website and entering the dog’s registered name in the search bar.
The German Shorthaired Pointer Club of America recommends the following health tests for German Shorthaired Pointers (1):
Dogs who have completed all of the health screening recommended by the GSPCA are assigned a CHIC identification number. It is important to note that a CHIC number only indicates the required tests have been performed, not passed. Always confirm the results of each test on the OFA website.
Source: OFA Website (2)
Common Health Problems Affecting German Shorthaired Pointers
Hip Dysplasia
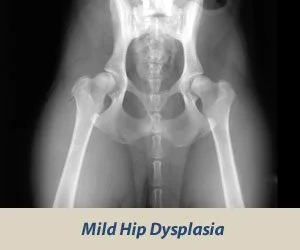
Canine Hip Dysplasia is a heritable disease that afflicts millions of dogs each year. It is caused by the the faulty development of the hip, causing excessive wear to joint cartilage. Excessive hip joint laxity can lead to osteoarthritis (OA), stiffness, and a diminished quality of life. (3) Screenings for Hip Dysplasia can be done through the Orthopedic Foundation for Animals (OFA) or through a PennHip exam.
OFA Screening: OFA’s screening process involves sending x-rays taken by a veterinarian to OFA for grading and certification. These X-rays are used to classify hips into seven different categories: Excellent, Good, Fair (all within Normal limits), Borderline, and then Mild, Moderate, or Severe (the last three are considered Dysplastic). The hip grades of excellent, good and fair are within normal limits and are given OFA numbers. Radiographs of borderline, mild, moderate and severely dysplastic hip grades are not passed. OFA hip testing must be performed when the dog is 24 months of age or older. (4)
PennHIP: PennHIP provides a numeric measure of how tight the hip joint is, which is predictive of future arthritis. For the PennHIP exam three radiographs are taken to measure the laxity of the hip joint. A score between 0-1 is assigned, with 0 being very tight hips and 1 being very loose. PennHIP testing is accurate in puppies as young as 16 weeks of age.
X-ray of Hip Dysplasia in a young dog. There is increased laxity of the joint causing a separation of the femoral head (ball) from the acetabulum (socket) and there is already some arthritis forming along the femoral neck. (5)
Radiographs (X-rays) of a normal dog’s pelvis and hips. The head of the femur (arrow) is seated deeply within the acetabulum, indicating excellent hip joint congruity. (5)
Source: OFA Website (4)
Elbow Dysplasia
Elbow dysplasia is a general term used to identify an inherited polygenic disease in the elbow. (6) Elbow dysplasia develops from one or a combination of these abnormal conditions:
Abnormalities involving the medial coronoid of the ulna (FCP). One of the two small bony protrusions on the end of the ulna develops a crack and separates from the rest of the bone.
Osteochondritis of the medial humeral condyle in the elbow joint (OCD). OCD refers to an abnormal development of the cartilage on the end of a bone in the joint. It is an inflammatory condition that occurs when the diseased cartilage separates from the underlying bone.
Ununited anconeal process (UAP). Growth plates are found at the ends of the bones. When a dog reaches puberty the growth plates close, fusing the parts of the bone together. If the anconeal projection of bone on the ulna doesn’t fuse to the rest of the ulna, it causes UAP. (7)
Early diagnosis of elbow dysplasia in dogs is important because you want to treat the condition before it causes osteoarthritis in the dog’s joint. The lameness associated with elbow dysplasia can be intermittent and mild for quite some time, which makes it a difficult disease to diagnose promptly. This is why using X-rays to screen our breeding dogs at or after 24 months of age is so important. Elbows are only passed if no signs of osteoarthritis are evident. Passing elbows are not given a grade, they are simply marked as “normal” in the OFA database.
Source: OFA Website (6)
Cardiac Disease
Subaortic stenosis (SSA) is the most common congenital heart disease in larger breeds of dogs. The purpose of the OFA’s cardiac screening process is to identify dogs which are phenotypically normal prior to use in a breeding program. (8) For the purposes of the registry, a phenotypically normal dog is defined as:
One without a cardiac murmur.
One with an innocent heart murmur that is found to be otherwise normal by virtue of an echocardiographic examination which includes Doppler studies.
Congenital heart conditions can be identified in young puppies, but dogs may not be certified by the Orthopedic Foundation for Animals as free of heart disease until after they are 12 months old. To receive a CHIC number, they must be screened after 24 months of age. Congenital heart disease in dogs is a malformation of the heart or great vessels. The lesions characterizing congenital heart defects are present at birth and may develop more fully during perinatal and growth periods. Many congenital heart defects are thought to be genetically transmitted from parents to offspring; however, the exact modes of inheritance have not been precisely determined for all cardiovascular malformations. (8)
You can obtain two levels of OFA certification for your dog:
Detection of a heart murmur with a stethoscope (Auscultation) OR
Ultrasound study of the heart (Echocardiogram)
Option 1, listening for a heart murmur, has historically been the method of choice for detecting heart disease. However, not all heart conditions are associated with heart murmurs. Therefore, with this method alone many dogs with heart disease go undetected. This obviously can impact the individual dog as their heart disease can’t be addressed appropriately. But, on a larger scale this can have a huge impact on a breeding program as genetic heart disease can continue to be passed on to offspring.
Option 2, an echocardiogram, is the gold standard for diagnosing heart disease, whether it is congenital or acquired, and is becoming the method of choice for certification by many breeders and individual pet owners. An echocardiogram is an ultrasound study of the heart that allows the cardiologist to look directly at the heart and determine if heart disease is present, even in the absence of a heart murmur. (21)
Eye Disease
OFA Companion Animal Eye Registry (CAER) exams are ophthalmic examinations performed by American College of Veterinary Ophthalmologist (ACVO) Diplomates, to assess dogs for the presence or absence of observable hereditary eye disease. (9) Dogs with normal exam results will receive OFA eye certification numbers that are valid for one year. Eye certifications are an important part of the routine health screenings practiced by responsible dog breeders to produce healthy puppies.
There are eye diseases in the dog for which there is evidence of a genetic or heritable cause. The American College of Veterinary Ophthalmologists has listed ten of these diseases as automatic “fails” (this means the affected dog is ineligible to receive an eye certification) because of the significance of the condition to vision and/or the very strong evidence of heritability. (9) Distichiasis, Entropion, and Persistent Pupillary Membranes (PPMs) are three conditions to be particularly mindful of in the German Shorthaired Pointer. (23) For a full list of eye diseases known to affect the breed, click here.
Cone Degeneration
Cone degeneration is an early onset, progressive disease of the retina resulting in loss of vision in day light (“day blindness”) typically beginning at about 8 to 12 weeks of age. Complete degeneration of retinal cone cells (and complete blindness in day light) is seen by the time affected dogs become adults. Typically, affected dogs become increasingly photophobic as exposure to bright light is irritating and painful. Though these dogs can still see in dim light conditions, it is easy to see how this disease could decrease an active dog’s overall quality of life. (10)
Cone Degeneration is screened for with a genetic test. Tests results will come back as clear, carrier, or affected. Cone degeneration is inherited in an autosomal recessive manner in dogs, meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. (11)
Dogs with N/N genotype will not have these types of cone degeneration.
Dogs with N/CD1 or N/CD2 genotype will not have these types of cone degeneration, but are carriers. They will transmit a cone degeneration variant to 50% of their offspring. Matings between two carriers are predicted to produce 25% cone degeneration-affected puppies.
Dogs with CD1/CD2, CD1/CD1, or CD2/CD2 genotype will have cone degeneration, an inherited disease that causes day-blindness. (22)
Autoimmune Thyroiditis
With Hypothyroidism, the thyroid gland is not making enough of a hormone called thyroxine that controls metabolism (the process of turning food into fuel). Hypothyroidism causes a wide variety of symptoms, but is often suspected in dogs that have trouble with weight gain or obesity and suffer from hair loss and skin problems. The good news is this disease isn’t life-threatening, it’s easy to diagnose with a blood test, and it’s fairly easy and inexpensive to treat. Treatment is typically a thyroid supplement taken daily. (12)
Autoimmune thyroiditis is the most common cause of primary hypothyroidism in dogs. The disease has variable onset, but tends to clinically manifest itself at 2 to 5 years of age. Dogs may be clinically normal for years, only to become hypothyroid at a later date. The marker for autoimmune thyroiditis, thyroglobulin autoantibody formation, usually occurs prior to the occurrence of clinical signs. Therefore, periodic retesting is recommended.
The majority of dogs that develop autoantibodies (antibodies that mistakenly target and react with a dog’s own tissues or organs) have them by 3 to 4 years of age. Development of autoantibodies at any time in the dog’s life is an indication that the dog most likely has the genetic form of the disease.
An OFA number will be issued to all dogs found to be normal at 12 months of age. Ages will be used in the certification process since the classification can change as the dog ages and the autoimmune disease progresses. It is recommended that reexamination occur at ages 2, 3, 4, 6, and 8 years.
Source: OFA Website (12)
Von Willebrand Disease
Von Willebrand disease type II is a moderate to severe blood clotting disorder characterized by an inability to clot appropriately after trauma or surgery. Severity of this disease varies greatly from dog to dog. Some dogs will spontaneously bleed from the nose or mouth or have blood in urine or stool, while other affected dogs will remain undetected until a traumatic event or surgery when severe bleeding occurs. Breeding females may also have excessive bleeding associated with heat cycles. Surgical procedures should not be performed on affected dogs without ready access to blood products for transfusion. (10) Age of onset can vary, with some dogs only becoming obvious “bleeders” later in life. Without medical intervention, uncontrolled bleeding can result in death. (13)
In many breeds, the presence of one abnormal vWF gene appears sufficient to cause abnormal and variable bleeding, an expression pattern referred to as "incomplete dominance". (14) Breeds with Type 2 (including the GSP) and Type 3 VWD appear to have recessive expression patterns. Clinically affected dogs usually have 2 abnormal vWF genes, inherited from both dam and sire. Genetic test results will come back as clear, carrier, or affected:
Dogs with N/N genotype will not have Von Willebrand disease Type 2 and cannot transmit this variant to their offspring.
Dogs with N/vWF genotype are unlikely to develop Von Willebrand disease Type 2, but are carriers. They may transmit this variant to 50% of their offspring. Matings between two carriers are predicted to produce 25% Von Willebrand disease Type 2-affected puppies.
Dogs with vWF/vWF genotype are affected and develop Von Willebrand disease Type 2. They will transmit this variant to all of their offspring. (15)
Degenerative Myelopathy (DM)
Degenerative myelopathy is a late onset, progressive neurological disease similar to amyotrophic lateral sclerosis (Lou Gehrig’s disease) in people. Dogs affected with this condition do not usually show clinical signs of disease until around 8-9 years of age. Dogs most commonly present with weakness in the hind limbs that is often mistaken as arthritis, vertebral disease or hip dysplasia by owners. Dogs with this condition are typically free of pain, but the weakness progresses forward on the body, leading to front limb weakness, urinary and fecal incontinence and the inability to walk. The disease typically progresses rapidly after initial signs appear and many dogs are unable to walk within 6 months to 2 years after initial presentation. The late age of onset makes selective breeding in the absence of genetic testing difficult because affected dogs typically do not show clinical signs until long after they have been bred and passed along the causal mutation to offspring. (10)
Degenerative myelopathy is inherited in an autosomal recessive manner with incomplete penetrance, meaning that if the dog receives two copies of the mutated gene (one from each parent) they might develop the disease. Genetic test results will come back as clear, carrier, or affected:
Dogs with N/N genoytpe will not have degenerative myelopathy and cannot transmit this variant to their offspring.
Dogs with N/DM genotype will not have degenerative myelopathy, but are carriers. They will transmit this variant to 50% of their offspring. Matings between two carriers are predicted to produce 25% degenerative myelopathy-affected puppies.
Dogs with DM/DM may have degenerative myelopathy, a disabling condition, and will transmit this variant to all of their offspring. (16)
Epilepsy
Inherited or idiopathic epilepsy (IE) is a common canine disease that causes repeated seizures. It is the most common neurological disease seen in dogs, and is the one of the top three overall health concerns for many dog breeds. (17) IE must be differentiated from secondary epilepsy in which there is an underlying cause such as brain tumor, brain malformation, brain infection, low blood sugar, low blood calcium, or liver failure. Determination of an appropriate treatment regimen for canine epilepsy depends on an accurate diagnosis of the type and cause of seizures.
A large number of genetic mutations have been associated with epilepsy in both humans and mice. In humans, the inheritance of epilepsy is generally complex, meaning that it involves interactions of one or more genes with each other as well as potentially with environmental factors. This is likely true of epilepsy in dogs as well. (18) However, currently there is no genetic test to identify carriers of the disease or affected dogs.
Typically dogs affected with IE will have their first seizure by three years of age. Most IE is considered to have a genetic basis. It is difficult for breeders to select against the disease because of the late onset and the inability to identify asymptotic carriers through genetic screening. Since the most common mode of inheritance for IE in dogs is either simple autosomal recessive or polygenic recessive it will be very difficult to eliminate carriers from breeding until conclusive genetic tests are developed. (17)
Epilepsy is known to affect Shorthairs. One survey of diagnosed dogs found that they are the fourth most affected breed. (17) Though this survey had some limitations, it does highlight how prevalent the issue is. Breeders must conduct careful pedigree research when pairing dogs to do their best to breed away from the disease. Breeding older unaffected dogs is one way to help limit the disease, as the majority of dogs with IE will seize early in life.
Cancer
Almost half of dogs over the age of 10 will develop cancer. Dogs can develop many of the same cancers as humans, and with very similar symptoms. A hereditary cancer predisposition syndrome gives the affected individual a tendency to develop cancer. These syndromes are due to a mutation in a gene that suppresses tumor growth. (19) Cancer, for the most part, is multifactorial. That means there are genetic and environmental influences. However, hereditary cancer predisposition syndromes have a much greater genetic influence than environmental influence when it comes to the risk of developing cancer.
Cancer can take many forms and can affect the blood, bone, or body tissues. An accurate diagnosis is critical to provide the most appropriate treatment and the best prognosis. Dogs fall victim to the following types of canine cancers (20):
Lymphoma is one of the most common cancers seen in dogs, accounting for 20% of all canine cancers. Dogs are two- to five-times more likely than people to develop lymphoma, which can affect any breed at any age. Lymphoma appears most often as swollen lymph nodes under the jaw, in front of the shoulders, or behind the knees. Occasionally, lymphoma may attack lymph nodes in the chest or abdomen, which makes breathing difficult and can cause vomiting and diarrhea. Lymphoma is generally considered treatable, depending on the type, and multidrug chemotherapy gives favorable results. Dogs who respond well to chemotherapy usually enjoy a good quality of life for the next 12 to 18 months of remission.
Mast cell tumors typically form on the skin, can vary from relatively benign to extremely aggressive, and often spread to other parts of the body. Mast cells are immune cells associated with allergies and usually are easily identified with a fine-needle aspirate. A pet suffering from a mast cell tumor will often show signs of vomiting, diarrhea, and loss of appetite. Surgery is required to excise the entire mast cell tumor. Often, chemotherapy and radiation therapy are also indicated for severe grades of tumor.
Osteosarcoma is the most common bone cancer in dogs, occurring most frequently in large and giant breeds. Osteosarcoma routinely attacks the long bones in the limbs—but can affect any bone—and progresses rapidly, spreading to the lungs, lymph nodes, and other bones. Pet owners notice swelling, lameness, or pain in the affected limb in the beginning stages. Since this disease is so aggressive and spreads extremely rapidly, the recommended course of treatment often is amputation of the affected limb followed by chemotherapy to treat metastases. Sadly, fewer than 10% of dogs who undergo this gold-standard treatment live longer than three years.
Melanoma is one of the most common oral cancers seen in dogs and frequently is seen in breeds with dark tongues and gums. These tumors are composed of darkly pigmented cells and can be found anywhere on the body. A malignant melanoma that develops in the oral cavity often has spread throughout the body by the time it’s first noticed and, unfortunately, is incurable. Complete surgical removal is difficult, radiation therapy is ineffective against metastasized cells, and chemotherapy is ineffective against this type of cancer. Melanoma appears to respond to immune-based therapies and several treatments are under development.
Mammary gland carcinomas are the most common tumors in unspayed female dogs, but often are overlooked since they usually appear as small nodules around the nipple. Unfortunately, the nodule may quickly grow into a large, painful tumor that can ulcerate and become an open wound. Approximately 50% of these tumors are malignant, but they can be cured with surgical removal if the cancer has not metastasized. Fifty percent of malignant masses will be fatal.
Hemangiosarcoma is a form of cancer that develops from cells that line the blood vessels, most commonly attacking the spleen but also the liver, heart, and skin. Dogs with splenic tumors seldom display signs of illness until the tumor ruptures and they go into shock caused by extreme blood loss, with pale gums, sudden weakness, and labored breathing. Emergency surgery is required to stem the blood loss, followed by chemotherapy. Sadly, this disease rarely gives warning signals until it has progressed into the later stages and treatment often is too late.
Although there are currently not many tests available for cancer predispositions in canines, this specific sub-specialty of canine genetic testing will only increase with time. This is especially true as knowledge of these diseases expands with veterinary research. Currently breeders rely on pedigree research and longevity to help reduce the incidence of hereditary cancers in the dogs they produce.
Lupoid Dermatosis (LD)
Lupoid Dermatosis (LD) is a serious degenerative disease that has been described in German Shorthaired Pointers and Vizslas. Clinical signs become apparent before one year of age. Although LD seems to only affect the skin at first, it can quickly progress to attacking the spleen, lymph nodes, and kidneys. Dogs soon develop lesions all over their body and may be unable to get up due to joint pain and swelling. Symptoms such as a rash, thickened skin, and hair loss are some of the first indicators of this disease. Because of this, it is imperative you take your GSP to see a veterinarian if they have any unexplained skin conditions.
LD is a monogenic (controlled by one gene) disease inherited in an autosomal recessive manner, meaning that a dog must receive two copies of the mutated gene (one from each parent) to develop the disease. (24) Dogs with LD have dramatically shortened life expectancies and are generally humanely euthanized upon diagnosis. The disease is progressive and ultimately fatal. No successful treatment has been reported. (25)
Sources
German Shorthaired Pointer Club of America. (n.d.). CHIC. Retrieved from https://www.gspca.org/Health/CHIC-Pop.html
Orthopedic Foundation for Animals. (n.d.). German Shorthaired Pointer CHIC. Retrieved from https://ofa.org/chic-programs/browse-by-breed/?breed=GSP
Donges, J. (2015, February 27). Pioneering the Diagnosis of Canine Hip Dysplasia. Retrieved from https://www.vet.upenn.edu/about/news-room/bellwether/bellwether-magazine/bellwether-winter-2015/pennhip-article
Orthopedic Foundation for Animals. (n.d.). What is Canine Hip Dysplasia? Retrieved from https://ofa.org/diseases/hip-dysplasia/
Texas A&M University Veterinary Medical Teaching Hospital. (n.d.). Canine Hip Dysplasia. Retrieved from https://vethospital.tamu.edu/small-animal/orthopedics/orthopedic-services/canine-hip-dysplasia/
The Orthopedic Foundation for Animals. (n.d.). The Three Faces of Elbow Dysplasia. Retrieved from https://ofa.org/diseases/elbow-dysplasia/
Meyers, H. (2022, April 20). Elbow Dysplasia in Dogs. Retrieved from https://www.akc.org/expert-advice/health/elbow-dysplasia-dogs/
Orthopedic Foundation for Animals. (n.d.). Cardiac Disease. Retrieved from https://ofa.org/diseases/cardiac-disease/
Orthopedic Foundation for Animals. (n.d.). Companion Animal Eye Registry (CAER) Overview. Retrieved from https://ofa.org/diseases/eye-disease/
Carl, C. (2014, May 15). Inherited Diseases of the German Shorthaired Pointer. Retrieved from https://www.pawprintgenetics.com/blog/2014/05/15/inherited-diseases-german-shorthaired-pointer/
Palanova, A. (2016, March 15). The genetics of inherited retinal disorders in dogs: implications for diagnosis and management. Retrieved from National Library of Medicine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6042528/
Orthopedic Foundation for Animals. (n.d.). Thyroid. Retrieved from https://ofa.org/diseases/thyroid/
Williams, K., & Ward, E. (n.d.). Von Willebrand's Disease in Dogs. Retrieved from VCA Animal Hospitals: https://vcahospitals.com/know-your-pet/von-willebrands-disease-in-dogs
Cornell University College of Veterinary Medicine. (n.d.). Canine von Willebrand Disease. Retrieved from Animal Health Diagnostic Center: https://www.vet.cornell.edu/animal-health-diagnostic-center/laboratories/comparative-coagulation/clinical-topics/canine-von-willebrand-disease
UC Davis Veterinary Medicine Veterinary Genetics Laboratory. (n.d.). Von Willebrand Disease II (vWD Type 2). Retrieved from https://vgl.ucdavis.edu/test/vwd-type-2
UC Davis Veterinary Medicine Veterinary Genetics Laboratory. (n.d.). Degenerative Myelopathy (DM). Retrieved from https://vgl.ucdavis.edu/test/degenerative-myelopathy
Patterson, E. (2007). Clinical Characteristics and Inheritance of Idiopathic Epilepsy. Retrieved from https://www.vin.com/apputil/content/defaultadv1.aspx?id=3861258&pid=11243
American Kennel Club Canine Health Foundation. (n.d.). Understanding Canine Epilepsy. Retrieved from https://www.akcchf.org/canine-health/top-health-concerns/epilepsy/understanding-canine-epilepsy.html
Tschirgi, M. (2014, April 21). Inherited Cancers in Dogs. Retrieved from Pawprint Genetics: https://www.pawprintgenetics.com/blog/2014/04/21/inherited-cancers-dogs/
American Animal Hospital Association. (n.d.). Is My Dog At Risk For Cancer. Retrieved from https://www.aaha.org/your-pet/pet-owner-education/ask-aaha/canine-cancer/
Veterinary Cardiology Specialists. (n.d.). Heart Clinics. Retrieved from http://www.vetcardiologist.com/heart-clinic/
UC Davis Veterinary Medicine Veterinary Genetics Laboratory. (n.d.). Cone Degeneration. Retrieved from https://vgl.ucdavis.edu/test/cone-degeneration
European College of Veterinary Ophthalmologists. (n.d.). German Shorthaired Pointer. Retrieved from ECVO Manual: https://www.ecvo.org/media/germanshorthairedpointer.pdf
Penn Vet University of Pennsylvania. (n.d.). Lupoid Dermatosis (Exfoliative Cutaneous Lupus Erythematosus). Retrieved from PennGen: https://www.vet.upenn.edu/research/academic-departments/clinical-sciences-advanced-medicine/research-labs-centers/penngen/penngen-tests/genetic-tests/Detail/60/
Moriello, Karen A. (2020, January). Multisystemic and Metabolic Defects in Animals. Retrieved from Merck Veterinary Manual: https://www.merckvetmanual.com/integumentary-system/congenital-and-inherited-anomalies-of-the-integumentary-system/cutaneous-manifestations-of-multisystemic-and-metabolic-defects-in-animals